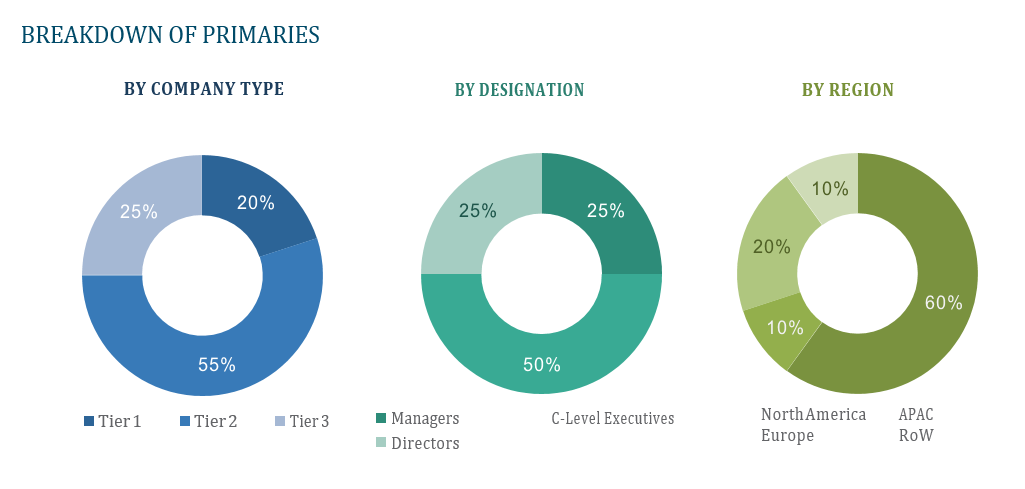
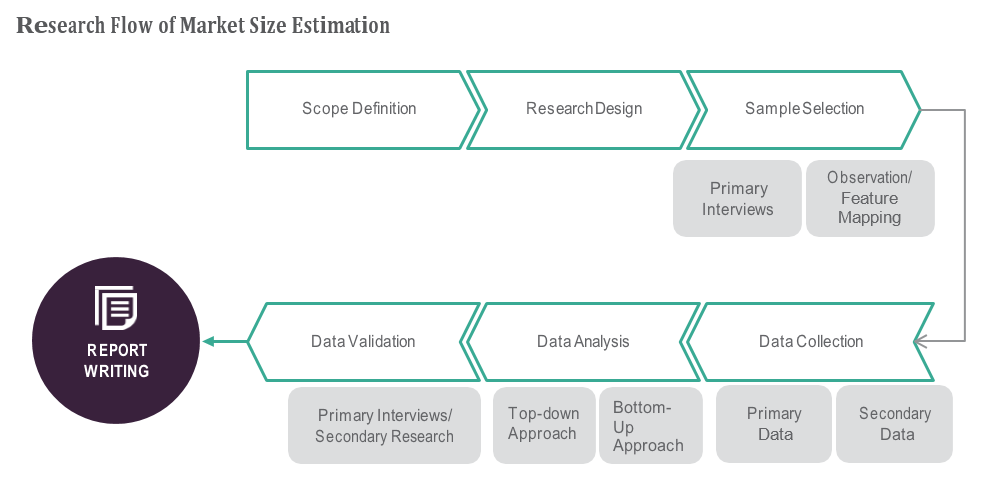
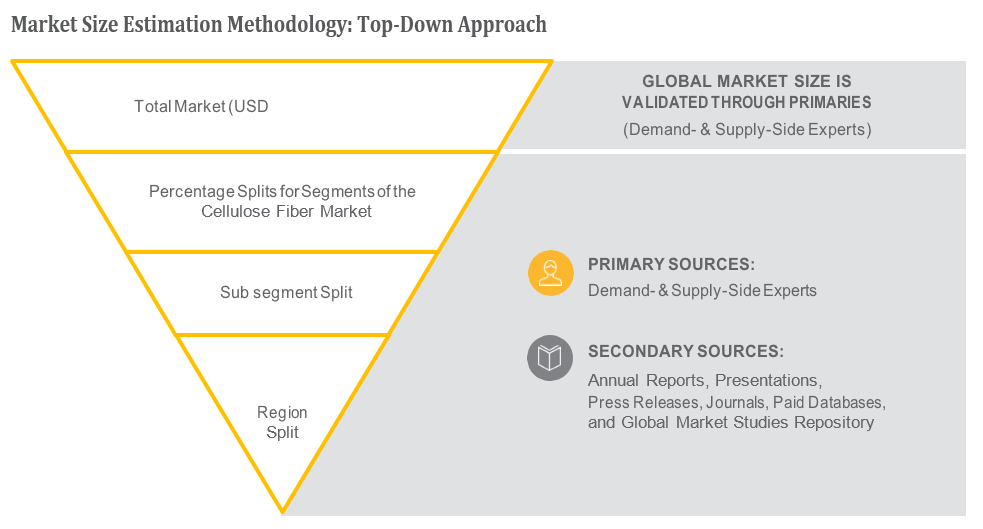
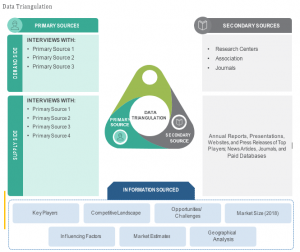
OVERVIEW
The Companion Diagnostics Market is currently valued at USD 7.5 billion in 2024 and will be growing at a CAGR of 12.6% over the forecast period to reach an estimated USD 13.6 billion in revenue in 2029. The companion diagnostics market is a pivotal component of precision medicine, revolutionizing healthcare by tailoring treatment to individual patients based on their genetic makeup, biomarker profiles, and specific disease characteristics. These diagnostic tests, often developed alongside therapeutic drugs, enable healthcare providers to identify patients who are most likely to benefit from a particular treatment, while minimizing potential adverse effects in those who may not respond. With advancements in molecular biology and technology, the companion diagnostics market continues to expand, offering increasingly sophisticated tools to guide personalized treatment decisions across a wide range of diseases, from cancer to infectious and autoimmune disorders.
One significant driver is the increasing prevalence of chronic and complex diseases, such as cancer, where personalized treatment strategies are becoming increasingly essential. Additionally, the expanding understanding of the genetic and molecular basis of diseases fuels the demand for companion diagnostics, as healthcare providers seek more targeted and effective treatment approaches. Moreover, regulatory initiatives supporting the integration of companion diagnostics into drug development and approval processes further propel market growth by fostering collaboration between pharmaceutical companies and diagnostic developers. Technological advancements, including next-generation sequencing and digital pathology, also play a crucial role in driving innovation within the companion diagnostics market, enabling more precise and comprehensive patient profiling. Finally, the growing emphasis on value-based healthcare and cost containment encourages the adoption of companion diagnostics to optimize treatment outcomes and reduce healthcare expenditures over the long term.
Table of Content
Market Dynamics
Drivers:
One significant driver is the increasing prevalence of chronic and complex diseases, such as cancer, where personalized treatment strategies are becoming increasingly essential. Additionally, the expanding understanding of the genetic and molecular basis of diseases fuels the demand for companion diagnostics, as healthcare providers seek more targeted and effective treatment approaches. Moreover, regulatory initiatives supporting the integration of companion diagnostics into drug development and approval processes further propel market growth by fostering collaboration between pharmaceutical companies and diagnostic developers. Technological advancements, including next-generation sequencing and digital pathology, also play a crucial role in driving innovation within the companion diagnostics market, enabling more precise and comprehensive patient profiling. Finally, the growing emphasis on value-based healthcare and cost containment encourages the adoption of companion diagnostics to optimize treatment outcomes and reduce healthcare expenditures over the long term.
Key Offerings:
Key offerings in the companion diagnostics market encompass a diverse range of products and services designed to facilitate personalized treatment approaches. These offerings typically include diagnostic tests that identify specific biomarkers or genetic variations associated with disease, enabling healthcare providers to stratify patients based on their likelihood of responding to particular therapies. Additionally, companion diagnostic assays may include molecular and genetic testing platforms, such as PCR, next-generation sequencing, and immunohistochemistry, which provide valuable insights into patient-specific disease characteristics. Alongside these tests, companion diagnostics providers often offer comprehensive support services, including assay development, validation, regulatory compliance, and clinical trial support, to pharmaceutical companies and healthcare institutions. Moreover, with the advent of digital health technologies, there is a growing emphasis on integrated data analytics solutions that leverage real-world patient data to inform treatment decisions and improve patient outcomes. Together, these key offerings empower healthcare stakeholders to deliver more precise, targeted, and cost-effective care, ultimately driving the advancement of personalized medicine.
Restraints :
The companion diagnostics market has a strong growth trajectory, but a number of obstacles prevent it from reaching its full potential. Complicated regulations and rigorous regulatory procedures frequently provide major obstacles to product development and market entrance, limiting patient access to companion diagnostic testing. Furthermore, companion diagnostics may not be widely used due to reimbursement issues and insufficient legislation, especially in areas where healthcare financing structures do not sufficiently encourage the use of personalised medicine approaches. Adoption may also be hampered by the high expense of the underlying technology and related diagnostic tests, particularly in healthcare facilities with limited resources. Companion diagnostics’ easy deployment and utilisation may also be hampered by interoperability problems and data protection concerns around its connection with electronic health records and other healthcare IT systems. To unlock the full potential of companion diagnostics in advancing personalised healthcare, industry stakeholders, policymakers, and regulatory bodies must work together to address these constraints. These efforts should focus on streamlining regulatory processes, improving reimbursement mechanisms, enhancing cost-effectiveness, and ensuring data security and interoperability.
Regional Information:
• In North America, the companion diagnostics market is propelled by robust healthcare infrastructure, strong research and development capabilities, and favorable regulatory frameworks that support innovation and commercialization. The region benefits from a high prevalence of chronic diseases, such as cancer, driving demand for personalized treatment solutions. Moreover, strategic collaborations between pharmaceutical companies and diagnostic developers bolster market growth, fostering the development and adoption of companion diagnostic assays. However, despite these favorable conditions, challenges such as reimbursement complexities and healthcare disparities across different states or provinces can hinder market penetration and access to companion diagnostics, particularly for underserved populations.
• In Europe, the companion diagnostics market is characterized by a well-established healthcare system, progressive regulatory policies, and a growing emphasis on personalized medicine initiatives. The region boasts a strong presence of key market players and academic research institutions, driving continuous innovation in companion diagnostic technologies. Additionally, initiatives such as the European Medicines Agency’s (EMA) qualification of novel methodologies and biomarkers further facilitate market expansion. Nevertheless, variations in reimbursement policies and fragmented healthcare systems across European countries can present hurdles to market access and adoption, necessitating harmonization efforts to ensure equitable access to companion diagnostics across the region.
• In Asia Pacific, the companion diagnostics market is fueled by rapid economic development, increasing healthcare expenditure, and a growing focus on precision medicine. Countries like China, Japan, and India are key contributors to market growth, driven by large patient populations and rising demand for personalized treatment approaches. Moreover, regulatory reforms aimed at expediting approval processes and promoting innovation in medical technologies stimulate market dynamics. However, challenges such as limited access to advanced diagnostic technologies in rural areas, disparities in healthcare infrastructure, and varying regulatory landscapes across different countries pose obstacles to market development and adoption of companion diagnostics in the region.
Recent Developments:
• In August 2023, Agilent Technologies, Inc. (US) received European IVDR Certification for Companion Diagnostic Assay.
• In August 2023, QIAGEN (Netherlands). received FDA approval for companion diagnostic to Blueprint Medicines’ AYVAKIT (avapritinib) in gastrointestinal stromal tumors.
Key Players:
Roche Diagnostics, Qiagen, Abbott Laboratories, Agilent Technologies, Thermo Fisher Scientific, Illumina, Myriad Genetics, bioMérieux, Danaher Corporation (including its subsidiary, Cepheid), and Exact Sciences Corporation.
1) What is the projected market value of the Companion Diagnostics Market?
– The Companion Diagnostics Market is expected to reach an estimated value of USD 13.6 billion in revenue by 2029.
2) What is the estimated CAGR of the Companion Diagnostics Market over the 2024 to 2029 forecast period?
– The CAGR is estimated to be 12.6% for the Companion Diagnostics Market over the 2024 to 2029.
3) Who are the key players in the Companion Diagnostics Market?
– Roche Diagnostics, Qiagen, Abbott Laboratories, Agilent Technologies, Thermo Fisher Scientific, Illumina, Myriad Genetics, bioMérieux, Danaher Corporation (including its subsidiary, Cepheid), and Exact Sciences Corporation.
4) What are the drivers for the Companion Diagnostics Market?
– The growing prevalence of chronic diseases like cancer necessitates personalized treatment strategies. The growing understanding of genetics drives demand for companion diagnostics. Regulatory initiatives, technological advancements, and value-based healthcare emphasize the importance of these diagnostics in drug development and approval processes, enabling more precise patient profiling and cost reduction.
5) What are the restraints and challenges in the Companion Diagnostics Market?
– The companion diagnostics market faces challenges such as regulatory complexities, reimbursement challenges, high costs, and data privacy concerns. These restraints hinder its full realization, especially in resource-constrained healthcare settings. To unlock the full potential of this technology, collaboration among stakeholders, policymakers, and regulatory bodies is needed to streamline processes, improve reimbursement, enhance cost-effectiveness, and ensure data security.
6) What are the key applications and offerings of the Companion Diagnostics Market?
– The companion diagnostics market offers products and services for personalized treatment, including diagnostic tests, molecular and genetic testing platforms, and comprehensive support services. These tools help healthcare providers stratify patients based on their response to therapies, provide insights into patient-specific disease characteristics, and leverage real-world patient data for improved treatment decisions.
7) Which region is expected to drive the market for the forecast period?
– North America is expected to have the highest market growth from 2024 to 2029
Why Choose Us?
Insights into Market Trends: Global Market Studies reports provide valuable insights into market trends, including market size, segmentation, growth drivers, and market dynamics. This information helps clients make strategic decisions, such as product development, market positioning, and marketing strategies.
Competitor Analysis: Our reports provide detailed information about competitors, including their market share, product offerings, pricing, and competitive strategies. This data can be used to inform competitive strategies and to identify opportunities for growth and expansion.
Industry Forecasts: Our reports provide industry forecasts, which will inform your business strategies, such as investment decisions, production planning, and workforce planning. These forecasts can help you to prepare for future trends and to take advantage of growth opportunities.
Access to Industry Experts: Our solutions include contributions from industry experts, including analysts, consultants, and subject matter experts. This access to expert insights can be valuable for you to understand the market.
Time and Cost Savings: Our team at Global Market Studies can save you time and reduce the cost of conducting market research by providing comprehensive and up-to-date information in a single report, avoiding the need for additional market research efforts.