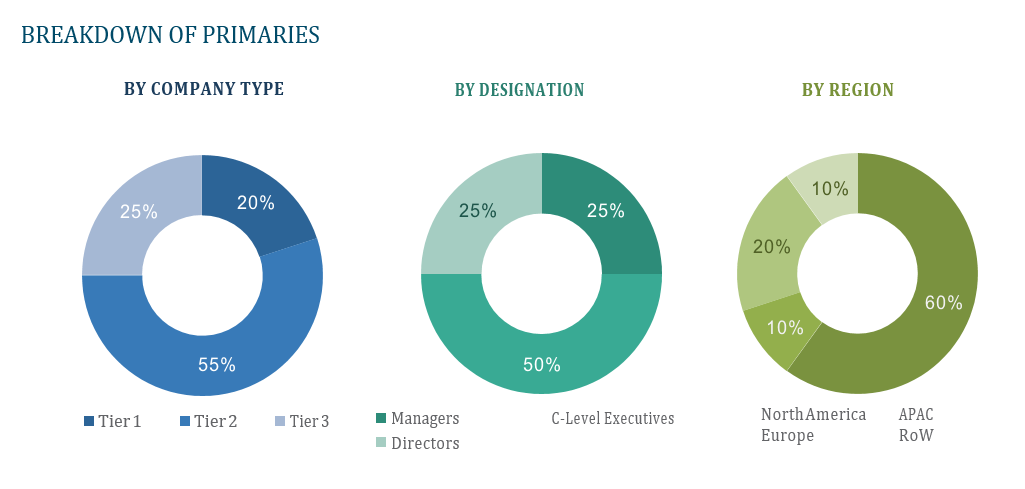
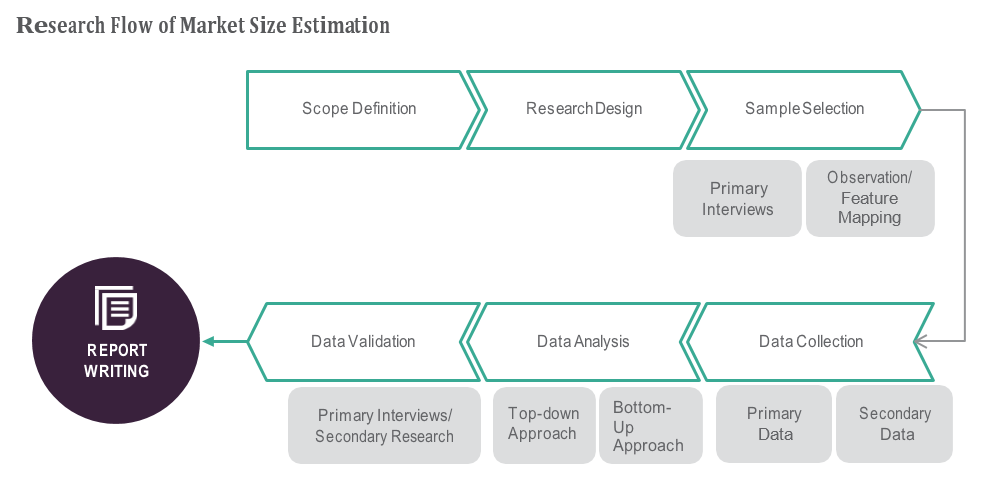
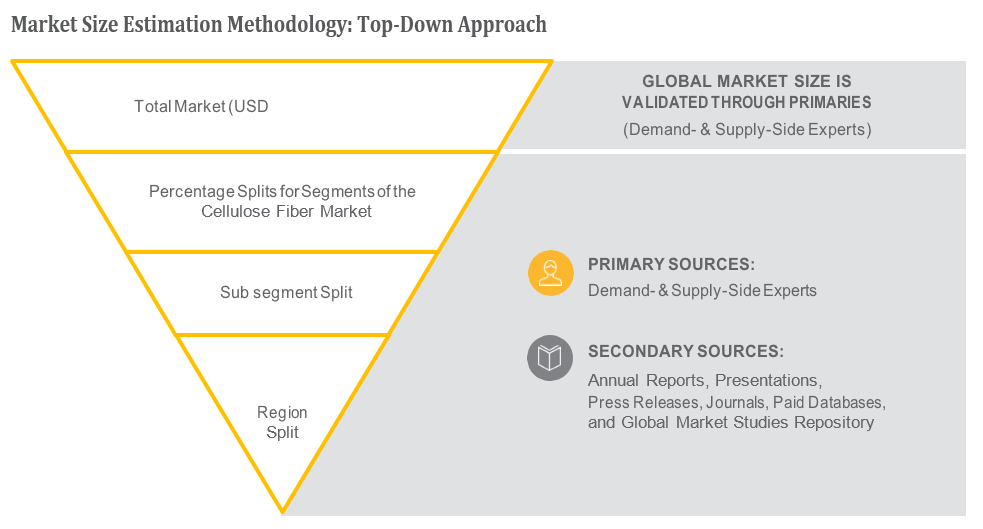
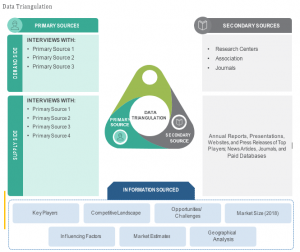
OVERVIEW
The Lateral Flow Assays Market is projected to grow significantly from USD 8.5 billion in 2024 to an estimated USD 13.2 billion by 2029, reflecting a CAGR of 9.2% during the forecast period. Lateral flow assays (LFAs) are simple, rapid, and cost-effective diagnostic tools used for the detection of specific analytes in complex mixtures such as blood, urine, saliva, and other fluids. These assays are widely used in various applications, including clinical diagnostics, food safety, environmental testing, and drug development. The increasing prevalence of infectious diseases, the growing demand for point-of-care testing, and advancements in LFA technology are driving the market’s growth.
The market’s expansion is also supported by rising healthcare expenditure, increased adoption of home-based diagnostic tests, and the integration of advanced technologies such as digital readouts and multiplexing capabilities in LFAs. However, challenges such as stringent regulatory requirements, limited sensitivity and specificity of some LFAs, and competition from alternative diagnostic methods need to be addressed to sustain market growth.
Geographically, North America and Europe dominate the lateral flow assays market due to their advanced healthcare infrastructure, significant investments in healthcare, and high adoption rates of innovative diagnostic technologies. The Asia Pacific region is also expected to witness substantial growth, driven by increasing healthcare expenditure, growing awareness of early disease detection, and the expanding healthcare sector.
Market Dynamics
Drivers:
The primary driver of the Lateral Flow Assays Market is the increasing prevalence of infectious diseases. Conditions such as COVID-19, influenza, and sexually transmitted infections (STIs) require rapid and accurate diagnostic solutions to control their spread and ensure timely treatment. Lateral flow assays offer several advantages over traditional laboratory-based testing, including quicker results, ease of use, and the ability to perform tests at the point of care. These benefits make LFAs an attractive option for both patients and healthcare providers. Additionally, advancements in LFA technology, such as the development of digital readouts and multiplexing capabilities, are enhancing the convenience and accuracy of these assays, contributing to market growth.
Another significant driver is the growing demand for point-of-care testing. Patients and healthcare providers are increasingly seeking diagnostic solutions that provide immediate results, enabling timely decision-making and treatment. Lateral flow assays are widely used in various medical fields, including emergency medicine, primary care, and home healthcare, to provide quick and reliable diagnostic information. The integration of advanced technologies, such as digital readouts and smartphone-based analysis, is further enhancing the capabilities of LFAs, enabling remote monitoring, real-time data sharing, and personalized treatment plans. The rising preference for point-of-care testing and the continuous advancements in LFA technology are driving the growth of the lateral flow assays market.
Key Opportunities
The Lateral Flow Assays Market presents numerous opportunities for growth and innovation, particularly in the development and integration of advanced technologies. One prominent opportunity lies in the increasing use of digital readouts and smartphone-based analysis in LFAs. Digital readouts enhance the accuracy and reliability of lateral flow assays by providing clear and quantifiable results, reducing the risk of human error. The integration of smartphone-based analysis with traditional LFA methods is expected to drive market growth by providing more reliable and efficient diagnostic solutions. Additionally, digital readouts and smartphone-based analysis can facilitate the development of connected diagnostic devices, improving data management and traceability.
The growing focus on expanding the applications of lateral flow assays presents another lucrative opportunity for the market. While LFAs are commonly used for infectious disease testing and pregnancy detection, there is increasing interest in exploring their potential for other medical conditions, such as cancer, cardiovascular diseases, and autoimmune disorders. Research and development efforts are being conducted to evaluate the safety and efficacy of LFAs for these indications. The expanding applications of lateral flow assays are expected to drive market growth by providing new diagnostic solutions for a broader range of medical conditions and treatment needs. Vendors that invest in research and development to explore new applications and improve existing LFA technologies are well-positioned to capitalize on this market trend.
Restraints:
One of the significant restraints in the lateral flow assays market is the stringent regulatory requirements. The development and deployment of LFA technologies require substantial investments in research, validation, and approval processes to ensure their safety, efficacy, and reliability. Compliance with regulatory standards, such as the FDA’s approval process and the European Union’s In Vitro Diagnostic Regulation (IVDR), is essential for market entry and commercialization. The complexity of obtaining regulatory approval and maintaining compliance with stringent standards can be a barrier to market growth. To overcome this restraint, vendors need to invest in quality assurance and regulatory affairs to navigate the complex regulatory landscape successfully.
Another challenge is the limited sensitivity and specificity of some lateral flow assays. While LFAs offer rapid and convenient diagnostic solutions, their performance can be affected by factors such as sample quality, environmental conditions, and operator variability. The limited sensitivity and specificity of some LFAs can result in false positives or false negatives, impacting the reliability of diagnostic results. To address this challenge, vendors need to focus on improving the accuracy and reliability of LFAs through advancements in assay design, material selection, and detection methods. Ensuring high sensitivity and specificity is critical for the widespread adoption and success of lateral flow assays in various diagnostic applications.
Regional Information:
- North America
North America remains a significant market for lateral flow assays, characterized by advanced healthcare infrastructure, high adoption rates of innovative diagnostic technologies, and substantial investments in healthcare research and development. The region’s strong presence of leading diagnostic device manufacturers and the high focus on enhancing patient care drive market growth. The increasing prevalence of infectious diseases, such as COVID-19 and influenza, is propelling the adoption of advanced LFA solutions in the region. Furthermore, stringent regulatory requirements and the need for rapid diagnostic testing encourage healthcare providers to invest in high-quality lateral flow assays. However, the high cost of advanced diagnostic devices and regulatory complexities remain challenges that need to be addressed to fully capitalize on the market potential.
- Europe
Europe leads in the adoption of lateral flow assays, driven by stringent regulatory requirements, significant investments in healthcare infrastructure, and a strong commitment to improving patient outcomes. The region’s focus on innovation and technological advancement fuels the demand for advanced diagnostic solutions. Countries like the UK, Germany, and France are at the forefront of implementing LFA technologies to enhance patient care capabilities and ensure compliance with regulatory standards. The European Union’s regulations, such as the In Vitro Diagnostic Regulation (IVDR), mandate stringent data protection and quality assurance measures, further driving market growth. However, economic uncertainties and the complexity of regulatory compliance necessitate strategic planning and risk management to navigate the market landscape effectively.
- Asia Pacific
The Asia Pacific region is expected to witness the highest growth rate in the lateral flow assays market due to rapid digital transformation, increasing healthcare expenditure, and the expanding healthcare sector. Countries like China, India, and Japan are investing heavily in advanced diagnostic solutions to support business growth and enhance patient care capabilities. The region’s expanding middle class and rising disposable incomes are also contributing to the increasing adoption of lateral flow assays in various sectors such as hospitals, diagnostic laboratories, and home healthcare. Governments in the region are implementing initiatives to promote digitalization and support the growth of the healthcare economy, further driving market growth. However, challenges related to regulatory compliance, fluctuating economic conditions, and the need for skilled healthcare professionals necessitate localized strategies and market insights for successful market penetration.
Recent Developments:
In August 2023, Quidelortho received authorization from the US Food and Drug Administration (FDA), allowing the company to market its new Sofia 2 SARS Antigen+ FIA.
Key market Players:
Frequently Asked Questions
1) What is the projected market value of the Lateral Flow Assays Market?
– The Lateral Flow Assays Market is expected to reach an estimated value of USD 13.2 billion in revenue by 2029.
2) What is the estimated CAGR of the Lateral Flow Assays Market over the 2024 to 2029 forecast period?
– The CAGR is estimated to be 9.2% for the Lateral Flow Assays Market over the 2024 to 2029.
3) Who are the key players in the Lateral Flow Assays Market?
– The primary drivers for the Lateral Flow Assays Market include the increasing prevalence of infectious diseases, the growing demand for point-of-care testing, and advancements in LFA technology. These factors are contributing to the rising demand for lateral flow assays. The integration of digital readouts and smartphone-based analysis is also driving market growth.
5) What are the restraints and challenges in the Lateral Flow Assays Market?
– The stringent regulatory requirements and limited sensitivity and specificity of some LFAs are significant challenges in the market. These factors can limit the adoption of advanced diagnostic technologies. Additionally, the complexity of obtaining regulatory approval and ensuring compliance with regulatory standards poses challenges that need to be addressed to ensure effective and secure diagnostic operations.
6) What are the key applications and offerings of the Lateral Flow Assays Market?
– Lateral flow assays are essential for providing rapid and accurate diagnostic results in various applications, including clinical diagnostics, food safety, environmental testing, and drug development. They support advanced diagnostic techniques, enabling immediate decision-making and treatment while ensuring compliance with regulatory standards. These solutions improve patient outcomes by offering convenient and quick diagnostic testing and facilitating early intervention. Additionally, LFAs are crucial in settings such as hospitals, primary care, emergency medicine, and home healthcare, enhancing the efficiency and effectiveness of patient management.
7) Which region is expected to drive the market for the forecast period?
– Asia pacific is expected to have the highest market growth from 2024 to 2029
Why Choose Us?
Insights into Market Trends: Global Market Studies reports provide valuable insights into market trends, including market size, segmentation, growth drivers, and market dynamics. This information helps clients make strategic decisions, such as product development, market positioning, and marketing strategies.
Competitor Analysis: Our reports provide detailed information about competitors, including their market share, product offerings, pricing, and competitive strategies. This data can be used to inform competitive strategies and to identify opportunities for growth and expansion.
Industry Forecasts: Our reports provide industry forecasts, which will inform your business strategies, such as investment decisions, production planning, and workforce planning. These forecasts can help you to prepare for future trends and to take advantage of growth opportunities.
Access to Industry Experts: Our solutions include contributions from industry experts, including analysts, consultants, and subject matter experts. This access to expert insights can be valuable for you to understand the market.
Time and Cost Savings: Our team at Global Market Studies can save you time and reduce the cost of conducting market research by providing comprehensive and up-to-date information in a single report, avoiding the need for additional market research efforts.